
Request Swabs
Samples available for evaluation via overnight shipping
Individually wrapped
Sterile Swabs - Ready to Use
Non-Sterile Swabs - Ready for Autoclave
Addressing The Shortage
In March 2020, Dr. Ramy Arnaout from Harvard Medical School put out a call to meet the swab shortage that was limiting COVID-19 testing.
We heard the call.
Abiogenix teamed up with FATHOM, HP and many others to iterate over several 3D printed designs. Using HP's Multi Jet Fusion technology, we quickly arrived at an optimized nasopharyngeal swab.
Working with clinicians on the front-lines, we designed the swab to be easier to use, and more comfortable than other 3D printed swabs.
Production is up and running at our Northern and Southern California facilities, producing autoclavable as well as ready-to-use swabs.
Over 1.4 million swabs produced to date.
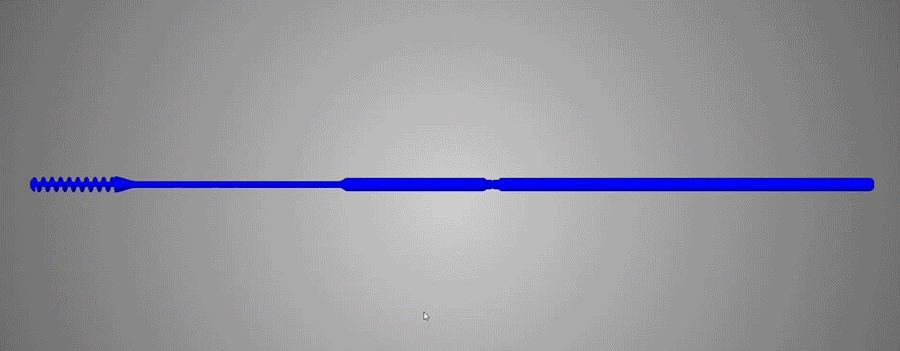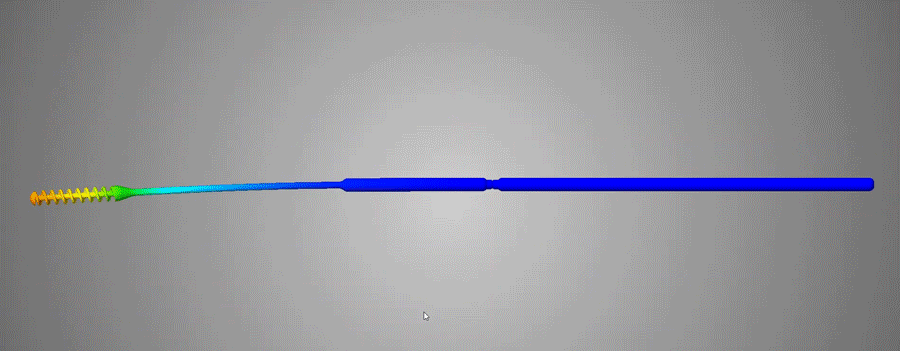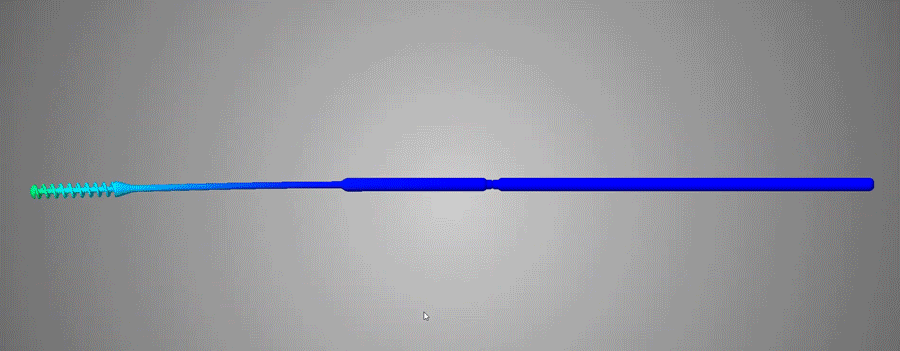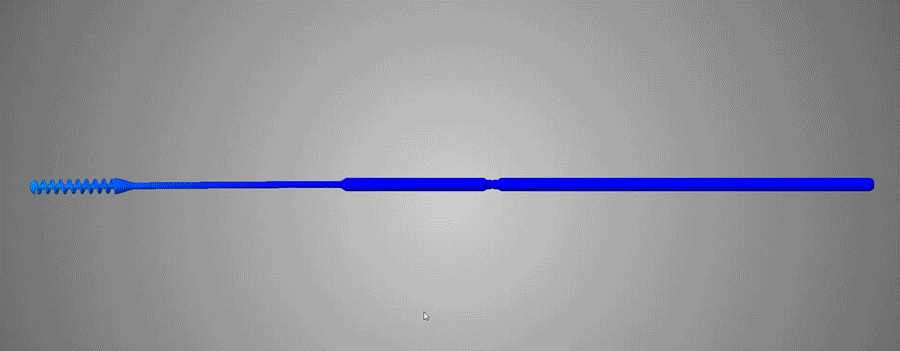
Flexible and durable

Abiogenix's FAST NP swabs passed tensile and torsion tests performed at Lawrence Livermore National Labs
Product Details
Ready
Sample swabs are available via overnight shipping
Manufacturing throughput of 100k daily
Individually wrapped
Sterile swabs ready for use
Non-sterile swabs ready for autoclave
Safe
Neck width diameters were optimized to not be too stiff, yet not be too flimsy, and were verified by clinicians to have the right “feel” of rigidity.
The breakpoint was optimized to not be too weak so as to buckle when clinicians pushed the swab against an obstruction. The breaking functionality was optimized to have the ability to perform single-handed breaking, by bending and then twisting.
Tested
The FAST Spiral NP swab was perfected with input from clinicians from Stanford Health Care, Harvard Medical School, and Seattle-area swab clinics.
The spiral design was selected as the most preferred novel swab (out of 150 designs) in a clinical study at Harvard-BIDMC.
It has passed expert review, collection sufficiency, PCR-compatibility, and concordance testing with flocked nasopharyngeal swabs.
*For initial diagnostic testing for CDC recommends collecting and testing an upper respiratory specimen. Nasopharyngeal specimen is the preferred choice for swab-based testing. Read CDC Article

Team

Core Team
Goutam Reddy, Founder Abiogenix Inc.
Rich Stump, CoFounder FATHOM

Contributors
Jenny Hu, Independent Mechanical Engineer, Medical Device Designer
Ehren Foss, Technical, Sales Solutions
Thomas Kluocek, Production Manager, FATHOM
Vince Corral, Quality Management, FATHOM
Anne Pauley, Mechanical Engineer, FATHOM
Carolina Aguirre, Content Strategist, FATHOM
Tony Slavik, Applications Engineer, FATHOM
Dr. Linda Barman, Stanford Health swab clinic
PA-C Thanh Khong, Stanford Health swab clinic
Lihua Zhao, Head of 3D Lab, HP Labs
Isabel Sanz, Additive Manufacturing Technical Consultant, HP
Jake Wright, Senior Research Scientist, HP Labs
Devin Mourey, Section Manager, Materials and Advanced Applications, HP
Sam Lazzara, Medical Device Quality Consultant
Coco Soodek, Seasongood Law

Independent Testing
Dr. Ramy Arnaout, Professor of Pathology, Harvard Medical School
Angela Tooker, Test Engineer, Lawrence Livermore National Laboratory
Laurence Stensland, Research Scientist, University of Washington





















